Targeted erlotinib for first line treatment of advanced non small cell lung cancer.
Erlotinib in the first line treatment of non small cell lung cancer.
We aimed to assess the safety and efficacy of erlotinib compared with standard chemotherapy for first line treatment of european patients with advanced egfr mutation.
A budget impact analysis.
Bajaj ps 1 veenstra dl goertz hp carlson jj.
A 64 year old woman receives the diagnosis of metastatic non small cell lung cancer nsclc which has progressed during treatment with carboplatin paclitaxel and bevacizumab.
Erlotinib at the standard oral daily dose of 150mg is approved for the treatment of unselected chemorefractory advanced non small cell lung cancer patients as well as maintenance therapy after first line chemotherapy.
1 university of washington pharmaceutical outcomes research and policy program department of pharmacy seattle wa 98195 usa.
Taraeva is indicated for.
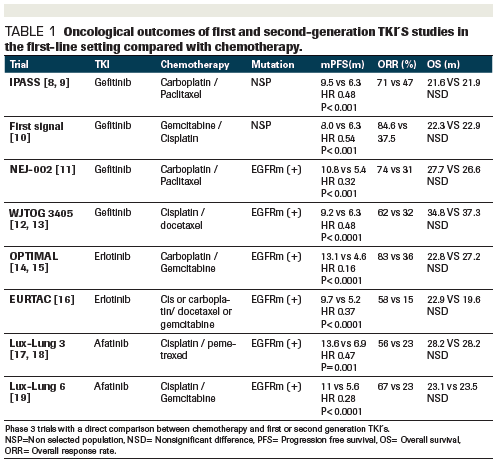
Activating mutations in egfr are important markers of response to tyrosine kinase inhibitor tki therapy in non small cell lung cancer nsclc.
The optimal study compared efficacy and tolerability of the tki erlotinib versus standard chemotherapy in the first line treatment of patients with advanced egfr mutation positive nsclc.
The cobas egfr mutation test detects mutations in the epidermal growth factor receptor egfr gene patients with advanced non small cell lung cancer nsclc who test positive for an egfr mutation may be eligible for erlotinib.
The us food and drug administration fda today approved a companion diagnostic test for erlotinib tarceva an oral cancer drug.
Erlotinib can prolong survival in patients with non small cell lung cancer after first line or second line chemotherapy.
Erlotinib has been shown to improve progression free survival compared with chemotherapy when given as first line treatment for asian patients with non small cell lung cancer nsclc with activating egfr mutations.
Interval 0 51 to 0 74.
Lung cancer of which non small cell lung cancer nsclc is the most common form remains the leading cause of cancer related mortality worldwide with many patients presenting with advanced disease at initial diagnosis.
Erlotinib is an orally administered small molecule inhibitor of epidermal growth factor receptor egfr tyrosine kinase.
The treatment of patients with metastatic non small cell lung cancer nsclc whose tumors have epidermal growth factor receptor egfr exon 19 deletions or exon 21 l858r substitution mutations as detected by an fda approved test receiving first line maintenance or second or greater line treatment after progression following.